FAST Research Series
The following are published posts in a series that summarizes lectures presented on Friday December 4th, 2015, the first day of the 2015 FAST Global Summit. This series will focused on research, pre-clinical, and future clinical trials. Be sure to join our mailing list so that you don't miss any of these exciting articles!




Summary of FAST 2015 Research Lectures
A great summary, revisiting some simple facts to refresh your memory to help put the subsequent posts in context. This also is a fantastic resource for explaining Angelman Syndrome to someone who has never heard of it before. Read here...
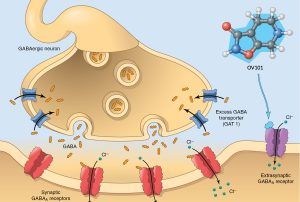
Ovid Therapeutics – OV101 as a potential therapeutic
Ovid Therapeutics is currently assessing the development of OV-101 for the treatment of Angelman Syndrome. Ovid will be working to select measures that reflect the burden of the syndrome on the patients and their families. This is likely to include sleep, motor, and behavioral measurements. Read more here...
Gene therapy for the treatment of Angelman Syndrome: Future clinical trials
Agilis Biotherapeutics recently announced that they have granted an Orphan Drug Designation status by the U.S. FDA for the indication of Angelman syndrome. This is a first for Angelman Syndrome. This post explains more about gene therapy and the approach that Agilis are taking. Read more...
The FIRE team of FAST turns on Ube3a in mice with Angelman Syndrome
FIRE team member Dr David Segal (pictured with Grad student Barbara Bailus) talks about the recently published paper "Protein Delivery of an Artificial Transcription Factor Restores Widespread Ube3a Expression in an Angelman Syndrome Mouse Brain". The future gets even more promising with Zinc Finger technology. Read more...