Ovid Therapeutics - OV101 as a potential therapeutic
The Development Of OV101 As A Potential Treatment For The Symptoms Of Angelman Syndrome
Ovid Therapeutics: Ovid therapeutics is currently assessing the development of OV-101 for the treatment of Angelman Syndrome. This summary summarizes FAST’s understanding of this potential clinical development program.
Background:
OV101, also known as Gaboxadol or THIP, is a small molecule that was derived from Muscimol, a naturally occurring compound isolated from the mushroom, Amanita muscaria. It is an oral medication that selectively works on extrasynaptic GABA receptors, i.e. outside the synapse, which is the very narrow space between two neurons where they communicate via neurotransmitters (chemical messengers). GABA is one such messenger that mediates “inhibitory neurotransmission or tonic inhibition” as discussed earlier, i.e. it dampens down excessive activity. See the Figure below from Ovid’s website. GABA acts on two types of GABA receptors: “synaptic” (between the nerves) and “extra-synaptic” (away from the nerve terminal)
In Angelman syndrome, loss of the UBE3A gene function leads to a build up of proteins that are normally marked by the cell machinery (“tagged”) for degradation and recycling by the Ube3a system. One such protein is the GABA transporter, GAT1, which is responsible for “mopping up” the GABA molecules after they are released. As a result these GABA transporters build up. In Angelman syndrome, it has been proposed that the excessive number of GABA transporters may lead to a relative deficiency of GABA in the extrasynaptic space. This in turn results in loss of activation of the extrasynaptic receptor and not enough “tonic inhibition”.
From a clinical perspective, what is important about this change is that this extrasynaptic receptor mediates “tonic inhibition”, the mechanism by which the brain filters incoming sensory information, and fine-tunes neuronal function. The loss of tonic inhibition has been proposed to be a fundamental mechanism underlying many of the symptoms of Angelman Syndrome. As shown in the diagram below, it is at this specific extrasynaptic receptor that OV101 works. Specifically, OV101 at clinically relevant doses, has selective binding to the GABAA receptors which have, uniquely, the delta subunit and which, as mentioned above, is only located on the extrasynaptic membrane.
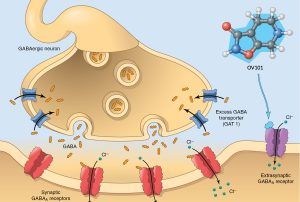 By binding to this receptor it is believed that OV101 restores tonic inhibition. OV101 is unlike any other molecule which acts on GABA receptors, not just structurally, but more importantly it is thought to be the only drug which selectively acts on the extrasynaptic receptor population, and hence is different from other, so called GABAergic medicines that families may be familiar with such as benzodiazepines (valium, clobazam, clonazepam, etc.), zolpidem (Ambien), anesthetic agents, and several anti-epileptic agents. These other drugs act in different ways on synaptic and in some cases, additionally, the extrasynaptic receptors.
The ultimate clinical result, as extrapolated from tests in the mouse models of Angelman syndrome, is hoped to be improvements in various aspects of the syndrome including motor function, sleep and behavior.
Here is our (FAST) understanding of important clinical information on OV-101, previously developed as gaboxadol:
By binding to this receptor it is believed that OV101 restores tonic inhibition. OV101 is unlike any other molecule which acts on GABA receptors, not just structurally, but more importantly it is thought to be the only drug which selectively acts on the extrasynaptic receptor population, and hence is different from other, so called GABAergic medicines that families may be familiar with such as benzodiazepines (valium, clobazam, clonazepam, etc.), zolpidem (Ambien), anesthetic agents, and several anti-epileptic agents. These other drugs act in different ways on synaptic and in some cases, additionally, the extrasynaptic receptors.
The ultimate clinical result, as extrapolated from tests in the mouse models of Angelman syndrome, is hoped to be improvements in various aspects of the syndrome including motor function, sleep and behavior.
Here is our (FAST) understanding of important clinical information on OV-101, previously developed as gaboxadol:
 By binding to this receptor it is believed that OV101 restores tonic inhibition. OV101 is unlike any other molecule which acts on GABA receptors, not just structurally, but more importantly it is thought to be the only drug which selectively acts on the extrasynaptic receptor population, and hence is different from other, so called GABAergic medicines that families may be familiar with such as benzodiazepines (valium, clobazam, clonazepam, etc.), zolpidem (Ambien), anesthetic agents, and several anti-epileptic agents. These other drugs act in different ways on synaptic and in some cases, additionally, the extrasynaptic receptors.
The ultimate clinical result, as extrapolated from tests in the mouse models of Angelman syndrome, is hoped to be improvements in various aspects of the syndrome including motor function, sleep and behavior.
Here is our (FAST) understanding of important clinical information on OV-101, previously developed as gaboxadol:
By binding to this receptor it is believed that OV101 restores tonic inhibition. OV101 is unlike any other molecule which acts on GABA receptors, not just structurally, but more importantly it is thought to be the only drug which selectively acts on the extrasynaptic receptor population, and hence is different from other, so called GABAergic medicines that families may be familiar with such as benzodiazepines (valium, clobazam, clonazepam, etc.), zolpidem (Ambien), anesthetic agents, and several anti-epileptic agents. These other drugs act in different ways on synaptic and in some cases, additionally, the extrasynaptic receptors.
The ultimate clinical result, as extrapolated from tests in the mouse models of Angelman syndrome, is hoped to be improvements in various aspects of the syndrome including motor function, sleep and behavior.
Here is our (FAST) understanding of important clinical information on OV-101, previously developed as gaboxadol:
- This drug has been trialed in over 3000 adults, predominantly in patients with primary insomnia. These studies showed that it was generally safe. However, the drug was never brought to market.
- This drug was evaluated in an AS mouse model (Egawa et al. 2012) and was found to show measureable improvement, particularly in motor function and ataxia (PubMed link).
- Remember mice are not people and measuring differences in various things in mice can be very difficult.
- Timeline: Ovid is estimating the clinical trial using OV101 will start the 2nd half of 2016. This will most likely be in adults first. A trial in younger patients will likely follow the adult trial. The timing for this pediatric trial is yet to be determined. Moreover, the specifics of the trial design, endpoints, inclusion and exclusion criteria are yet to be confirmed.
- Route: This is an oral pill, with either once or twice daily dosing.
- Location: This trial will start in the USA first, but will plan to move to other countries like Europe/Australia/etc.
- Ovid will work with various locations around the country and are aiming for East Coast, West Coast and Midwest locations (at least). No mention of where exactly.
- Ovid is passionate and committed in helping the Angelman community, and looks forward to building a long-term relationship to accomplish this goal.
- What will outcome measures be for this trial? Ovid will be working with the Angelman community, advisors, and key investigators to select measures that reflect the burden of the syndrome on the patients and their families. This is likely to include sleep, motor, and behavioral measurements.
- Who will be included? All genotypes of AS including deletion, mutation, UPD, and ICD, as long as there is a medical genetic diagnosis (e.g. karyotype, microarray, and methylation test). Ovid will consider helping to fund genetic testing for those that do not yet have a molecular diagnosis and have only been given a clinical diagnosis (more common in some of our older kids/adults).
- How will families apply to be enrolled in this clinical trial? Investigators who will be participating in the clinical studies will have the ultimate decision and responsibility for enrolling patients. Communication about the trial will be available through various channels including clinical trial information, media in general and major organizations. Keeping your eye on social media will prevent you from missing it. FAST will make this very clear.
- What is the plan for younger children? Must wait and talk to the FDA but want to accelerate this as fast as possible.
- Why was drug not approved for sleep? See above. Merck and its partner Lundbeck terminated the development program in 2007. In a press release, Merck and Lundbeck indicated that “Data from recently completed clinical studies suggest that the overall clinical profile for gaboxadol in insomnia does not support further development”
- What is chance of regression if you stop taking the drug? It is unknown. The proposed clinical trials will address this issue.
- Will Ovid seek Orphan drug status? Ovid’s current focus is in initiating the first study in Angelman Syndrome. Ovid will also consider all potential regulatory filings including but not limited to Orphan Drug Designation.
- What is the time frame for the trial to get to UK/EMA/Australia? Will organize in USA first and then consider these other areas.